In recent years, as the national policy on data center PUE (power usage efficiency) restriction continues to tighten, liquid cooling technology, as a recognized tool to reduce PUE, has become an industry focus technology. The latest document issued by four departments, including the Development and Reform Commission and the Ministry of Industry and Information Technology, requires that by 2025, the PUE value of large and super-large data centers be reduced to below 1.3, while liquid cooling can reduce the PUE value to about 1.1 to meet the policy requirements. In addition, liquid cooling can solve the problem of high-density heat dissipation and save construction costs, and also has the advantages of quiet and low noise, easy siting, and more adaptable to future development.
There are many requirements for liquid coolants for electronic applications, which vary by application type and may generally include
– Good thermophysical properties (high thermal conductivity and specific heat; low viscosity; high latent heat of evaporation for both applications)
– Low freezing and bursting points (sometimes burst protection at -40°C or lower is required for transportation and/or storage purposes)
– High boiling points (or low vapor pressure at operating temperature) required for single-phase systems; suitable boiling points for two-phase systems
– Good chemical and thermal stability over the life of the electronic system
– High flash point and self-ignition temperature (sometimes non-flammability is required)
– Non-corrosive to materials of construction (metals as well as polymers and other non-metals)
– No or only minimal regulatory restrictions (environmentally friendly, non-toxic, biodegradable).
– Economical.
Since in submerged liquid cooling technology, the coolant is in direct contact with the electronic product, there are strict requirements for the performance of the coolant such as insulation and heat transfer, and it is considered that the ideal submerged coolant needs to meet the following technical specifications.
(1) Weak insulator storage of electrical energy with a dielectric constant <2.5 (under 1 kHz conditions), allowing high frequency electronic components and connectors to be submerged in the coolant without significant loss of signal integrity.
(2) Excellent insulation properties with volume resistivity > 1 × 1012 Ω-cm and dielectric strength > 24 kV (2.54 mm gap).
(3) Low surface tension and low viscosity, with a kinematic viscosity of <50 cSt for liquids at the lowest operating temperature.
(4) the boiling point of dual-phase submerged coolant is generally 20~100 ℃; the boiling point of single-phase submerged coolant is generally greater than 100 ℃.
(5) excellent heat transfer properties, specific heat capacity ≥ 0.96 J/(g-K ), liquid thermal conductivity ≥ 0.06 W/(m-K).
(6) good material compatibility, high chemical stability, non-combustible, and no corrosion when in contact with electronic components.
(7) acute toxicity requirements LC 50 > 2000 mg/kg.
(8) Friendly environmental performance with zero ozone depletion potential (ODP) and global warming potential (GWP) <250 [3].
According to the current research experience, it has been shown that the indicators such as dielectric constant, GWP value, and insulation performance index are difficult to be satisfied simultaneously. Therefore, these indicators become the key indicators of success or failure in the development of submerged coolants.
Up to now, submerged coolants can be divided into the following four types according to their molecular structure characteristics: hydrofluoric saturated compounds, hydrofluoric unsaturated compounds, perfluoric saturated compounds and perfluoric unsaturated compounds, which can be further subdivided into hydrofluoric hydrocarbons, hydrofluoric ethers, hydrofluoric olefins, unsaturated hydrofluoric ethers, perfluoric alkanes, perfluoric amines, perfluoric polyethers, perfluoric olefins, perfluoric alkenyl amines, perfluoric alkenyl ethers, and other types.
2.1 Hydrofluorinated saturated compounds
Hydrofluoric saturated compounds submerged coolants mainly include hydrofluorocarbons and hydrofluoroethers, which are characterized by high GWP values and large dielectric constants. Generally speaking, the dielectric constant and GWP value of hydrofluorocarbons are high, and their insulation performance and environmental performance do not meet the technical specifications of ideal submerged coolants. The dielectric constants of hydrofluoroethers are all higher, all much higher than 2.5. The reason for this is that the molecules are more polar, which leads to an increase in their dielectric constants. At present, hydrofluoric ethers are mainly used in fields where the requirement of dielectric constant is not very demanding.
2.2 Hydrofluoric unsaturated compounds
Since saturated compounds such as hydrofluorocarbons and hydrofluoroethers have high GWP values and are not environmentally friendly, unsaturated compounds such as hydrofluoroolefins or unsaturated hydrofluoroethers can be obtained by introducing C=C or ring structures into the above structures, which can significantly enhance their reactivity with OH radicals, thereby reducing their GWP values and improving their environmental performance.
2.3 Perfluorinated saturated compounds
Due to the high dielectric constant of hydrofluorocarbons and hydrofluoric ethers and other hydrofluorosaturated compounds, they are not non-dielectric fluids.
The small radius of fluorine atoms and the concentration of negative charges result in fluorine having a low electron and atomic polarization rate. Therefore, one of the unique effects caused by fluorine substitution is to reduce the polarizability of the compound molecule, thus reducing the dielectric constant of the compound. It was concluded that the mass percent of fluorine and the molecular volume polarizability of a compound are the main factors affecting the dielectric constant, and it was shown that the higher the mass percent of fluorine, the lower the dielectric constant, and the lower the molecular volume polarizability, the lower the dielectric constant.
The introduction of fluorine atoms into the structures of hydrofluorinated saturated compounds such as hydrofluorocarbons and hydrofluoroethers with high dielectric constants, and the total substitution of hydrogen atoms in the structures to obtain perfluorinated saturated compounds such as perfluorocarbons or perfluoropolyethers, can reduce the molecular polarization rate and thus the dielectric constant.
2.4 Perfluorinated unsaturated compounds
Although perfluorinated compounds such as perfluorinated hydrocarbons, perfluorinated amines and perfluorinated polyethers have low dielectric constants, they can meet the requirements of ideal immersion from the technical index level. However, the GWP values of the above compounds are generally greater than 5000, which has a strong greenhouse effect. Therefore, the introduction of C=C structures or ring structures in perfluorinated compounds has become the main strategy to improve their environmental performance.
In order to meet the demand for fast and efficient heat dissipation in data centers, the foundation of big data and cloud computing, Quanzhou Yuji New Material Technology Co., Ltd. has developed a series of submerged fluorine-containing electronic coolants with independent intellectual property rights, such as FEC-50, FEC-80, FEC-110, FEC-140, FEC- 160 etc.
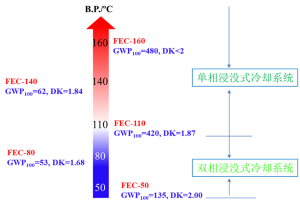
| FEC-50 | FEC-80 | FEC-110 | FEC-140 | FEC-160 | |
| Product properties | Liquid | Liquid | Liquid | Liquid | Liquid |
| Molar mass, g/mol | 300 | 362 | 450 | 512 | 600 |
| Liquid density, g/mL,25℃ | 1.5873 | 1.72(20℃) | 1.83 | 1.546 | 1.709±0.06 |
| Specific heat capacity, J/(kg K) | 1.2977 | 1.0465 | 0.865 | 1.4601 | 1.7113 |
| Thermal conductivity, W/(m k) | 0.07130 | 0.0709 | 0.06844 | 0.0701 | 0.0635 |
| Latent heat of vaporization, KJ/mol | 28.93±3.0 | 29.94 | 32.067 | 33.29±3.0 | 40.92±3.0 |
| Boiling point, ℃ | 47.1 | 83.6 | 107.2 | 108.5±40.0 | 158.6 |
| Saturated vapor pressure, mmHg (25°C) | 215 | 18.35 | / | 30.2 | 0.749 |
| Flammability | No | No | No | No | No |
| Dielectric constant (1kHz, 25°C) | 2.00 | 1.68 | 1.87 | 1.84 | <2 |
| ODP value | 0 | 0 | 0 | 0 | 0 |
| GWP100 | 135 | 53 | 420 | 62 | 480 |