Are you aware that when you drink water, you might be consuming TFA, a “forever chemical” known to potentially harm fertility and health? It may sound alarming, but recent research suggests this could be a reality.
The Escalating Presence of TFA in Water Alarm Experts
In May 2024, the European Pesticide Action Network (PAN Europe) released a study on Trifluoroacetic Acid (TFA) in water. The study revealed alarming levels of TFA in 23 surface water and six groundwater samples across ten EU countries, including France, Germany, Belgium, Spain, Austria, and the Netherlands.
According to the study, all water samples analyzed contained PFAS (per- and polyfluorinated substances). Over 98% of the total PFAS detected were TFA, and a concerning 79% of these samples had TFA concentrations exceeding the proposed limit of 500 ng/l for total PFAS, as outlined in the EU Drinking Water Directive set to be implemented in 2026.
However, this issue extends far beyond European borders. Dr. David Behringer, an environmental consultant, points out that China has witnessed a 17-fold surge in TFA levels in surface waters over a decade. Similarly, the United States has seen a sixfold increase in 23 years. In Germany, TFA levels in rainwater have increased fivefold in the last twenty years. A 2021 study revealed that over a two-year period, TFA levels in precipitation at eight monitoring sites in Germany were several times higher than they were a quarter-century ago and are expected to rise even further.
Scientists and environmental advocates are urgently calling for attention and regulatory measures to address the continuous rise of TFA.
So, what is TFA?
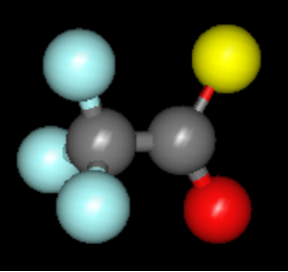
Figure 1 – Molecule of trifluoroacetic acid, TFA
Trifluoroacetic acid (TFA), chemical formula CF3CO2H (see Figure 1), is a colorless, vinegar-scented liquid that belongs to the large family of per- and polyfluoroalkyl substances (PFAS). TFA exhibits varying toxicity levels across different organisms and is often referred to as a ‘forever chemical’ due to its high resistance to natural degradation processes and its high mobility, which allows it to easily migrate into water systems over time.
In 2019, TFA was recognized as a persistent, mobile, and toxic (PMT) substance and added to ChemSec’s SIN List due to its concerning environmental.
Where does TFA come from?
TFA originates from various sources within the environment, some of which are natural, while others are the result of human activity.
Natural Sources
The primary natural source of TFA is believed to be undersea volcanic activity, accounting for an estimated 95% of the TFA currently found in the environment.
Human-Made Sources
Human-made TFA primarily comes from the atmospheric degradation of halogenated hydrocarbons, including hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These compounds were introduced as substitutes for ozone-depleting chlorofluorocarbons (CFCs) in applications like refrigeration, air conditioning, aerosol sprays, and heat pumps. Once released into the atmosphere, these substances degrade into TFA and fall to earth with rainwater.
Notably, HFOs, widely recognized as the fourth-generation ODS (Ozone-Depleting Substances) substitutes, were believed to be environmentally friendly due to their zero ozone-depleting potential and low Global Warming Potential (GWP). However, evidence shows that HFOs are no better than the previous generation of products, such as CFCs and HFCs, in terms of their capability to degrade into TFA. According to the 2022 Assessment Report of the UNEP Environmental Effects Assessment Panel, the atmospheric degradation of HFO-1234yf, HFO-1234ze (E), and HFO-1336mzz(Z) yields 100%, 2-30%, and 4-60% of TFA, respectively.
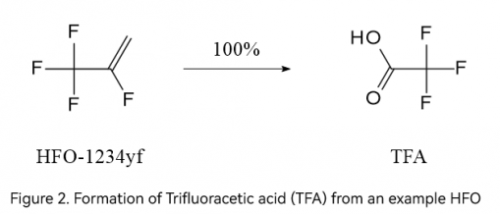
The debate over man-made TFA
The debate over the impact of man-made TFA is ongoing. Some experts, backed by the European Fluorocarbons Technical Committee representing the F-gas and chemicals industry, argue that the scale of man-made TFA production and its resulting environmental impact is inconsequential.
However, other researchers contend that the industrial contribution to TFA levels is significant, particularly concerning its rapid accumulation on land. They point out that the atmospheric degradation of HFCs and HFOs into TFA can occur in as little as two weeks, leading to its easy accumulation in terrestrial aquatic systems. In contrast, naturally formed TFA tends to concentrate in the deep ocean at a much slower rate.
Is TFA Globally Regulated?
The regulation of TFA has been a slow process, with only a handful of countries establishing regulatory limits. Denmark and Germany have set limits for TFA in drinking water, but the UK has not followed suit. In England, water companies have been tasked with assessing their drinking water sources for 47 types of PFAS, yet TFA is absent from this list.
The recent evidence of TFA increase in surface waters has underscored the urgent need for a comprehensive understanding and management of TFA’s environmental and health impacts. Some experts even call for the immediate stop of all applications leading to the release of TFA to the environment through the general PFAS restriction under REACH.
The Health and Safety Executive in Britain has labeled TFA as “a substance of concern” due to indications that it might cause developmental toxicity. Concurrently, the UK Environment Agency is preparing a focused program to test for TFA in surface and groundwater.
In a significant move, the German Federal Office for Chemicals (Bundesstelle für Chemikalien or BfC) announced in March 2024 its plan to inform the European Chemical Agency (ECHA) of its intention to propose linking reproductive toxicity to TFA. This initiative marks one of the first attempts to associate exposure to small quantities of TFA (concentrations of at least 0.1% to 0.3%) with adverse health effects.
Furthermore, the BfC aims to propose linking reproductive toxicity to sodium trifluoroacetate and other inorganic salts of TFA, as well as to suggest that inhaling TFA could be toxic.
Quanzhou Yuji Takes on the TFA Challenge with Innovative Solutions
In the hope of addressing the global TFA problem, Quanzhou Yuji Advanced Materials Co. Ltd. started researching new ODS substitutes many years ago. The R&D team at Quanzhou Yuji has spent countless hours to screening tens of thousands of candidate molecules to find alternatives to 4th-generation ODS substitutes (HFOs).
Headquartered in Quanzhou City, China, Quanzhou Yuji is dedicated to developing environmentally friendly fluorine-containing materials, including refrigerants, blowing agents, aerosol propellants, fire extinguishing agents, solvents, electronic gases, insulating gases, and immersion fluorinated electronic coolants.
In April 2024, Yuji introduced the concept of the “5th-generation ODS substitutes” at an industrial conference in China. This series of fluorocarbon compounds has extraordinary environmental friendliness, they have zero ODP, low GWP, and most importantly, they do not decompose into TFA in the atmosphere. This marks a significant milestone in the development of ODS substitutes.
Figure 3. Quanzhou Yuji introduced the “5th-generation ODS substitutes” at industrial conference.
On May 23, Yuji was honored with the ‘China Fluorosilicone Industry Innovative Enterprise’ award at the 8th China Fluorosilicone Industry Conference, recognizing the company’s commitment to technological advancement and environmentally friendly chemicals. Furthermore, Quanzhou Yuji has successfully scaled up production of the “5th-generation ODS substitutes” to the hundred-ton level and is excited about the positive impact this discovery will have on both the environment and humanity.

Figure 4. Yuji was honored with the ‘China Fluorosilicone Industry Innovative Enterprise’ award
References:
6.The United Nations Environment Programme (UNEP) (ed.) (no date) Environmental effects of stratospheric ozone depletion, UV … Available at: https://ozone.unep.org/system/files/documents/EEAP-2022-Assessment-Report-May2023.pdf