On October 14, 2024, the Belgian citizen group Grondrecht and climate organization Climaxi filed an appeal with the Council for Permit Disputes against new PFAS discharge standards approved for 3M Belgium.
These standards, set earlier this year, permit 3M to release extremely high levels of ultra-short-chain PFAS into the Scheldt River, including 15,000 ng/l for Trifluoroacetic acid (TFA) and 21,000 ng/l for PFPrA.
Climaxi claims these levels pose a serious risk to water quality and public health, as research shows that up to 98% of PFAS in drinking water comes from ultra-short-chain variants, which are toxic and persist in the environment.
Climaxi argues that 3M is aware of its pollution level and has the ability to remediate instead of merely moving the contamination from underground to the river. This appeal might be one of the first legal challenges concerning TFA in water. TFA, known as a “forever chemical,” has been found in groundwater across many EU countries. A May 2024 study by PAN Europe revealed troubling levels of TFA in samples from ten EU countries, with most exceeding the proposed limit of 500 ng/l outlined in the upcoming EU Drinking Water Directive.
TFA in water is escalating globally, with studies showing a 17-fold increase in China over the past decade, a sixfold rise in the U.S. over 23 years, and a fivefold surge in Germany’s rainwater in the last two decades.
Where does TFA in Water come from?
In addition to other sources, rising levels of TFA in water result from the atmospheric degradation of halogenated hydrocarbons, including HFCs and HFOs. These substances were developed as alternatives to ozone-depleting CFCs for applications such as refrigeration, air conditioning, aerosol sprays, and heat pumps. Once released into the atmosphere, they degrade into TFA, which ultimately returns to the earth via rainwater.
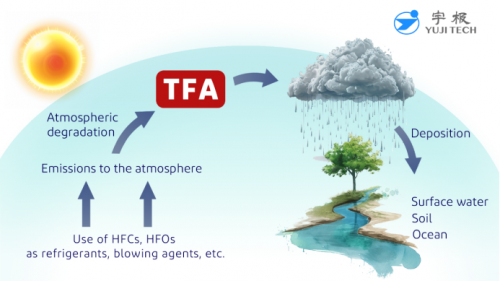
Figure 1-TFA formation from Atmospheric degradation of HFCs and HFOs
Quanzhou Yuji Takes on the TFA Challenge with Innovative Solutions
In April 2024, Yuji proposed the “5th-generation ODS substitutes.” These carefully selected fluorocarbon compounds do not decompose into trifluoroacetic acid (TFA) in the atmosphere, aiming to address the escalating global accumulation of TFA.